PRP
PLATELET-RICH PLASMA
Platelet-Rich Plasma - PRP
Hair restoration technique
Platelet-rich plasma has been used for years now to heal wounds in a variety of medical fields and Dr. Bisanga believes it may have significant benefits in hair transplantation, including healing in donor and recipient areas as well as improving post-op skin and hair physiology.
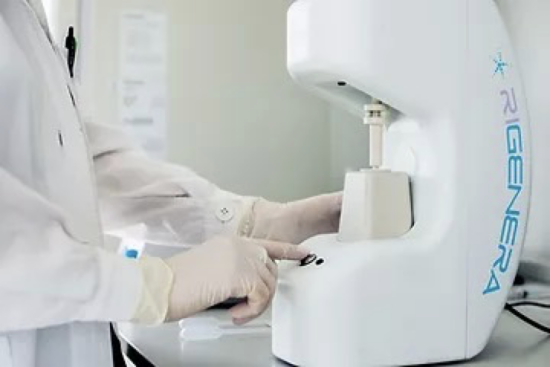
The approximate consistency of platelets in one cubic cm of blood is 250,000 and when concentrated with PRP it can increase to 1,000,000 – four times the normal concentration. Platelets are parts of the blood that contain the “healing” tools that are released when a wound is opened.
The concentrated nature of PRP and the fact it can be applied to isolated areas means that the platelets can react faster to improve coagulation, reduce bleeding, prevent fibrosis and hinder collagen production, resulting not only in accelerated rate of healing, but also improved skin tissue recovery which reduces scarring.
Platelets are also rich in proteins and may aid healing in both recipient placement sites for new grafts as well as in donor areas for either punch scarring (FUE surgery) or linear scars (strip surgery).
Protein growth factors should accelerate healing, in turn stimulating the formation of vessels and helping follicles to bond more quickly with the site, tissues and the cardiovascular system.
These proteins induce fibrosis and thus enhance the conversion of fibrinogen into fibrin (albeit to a lesser extent than naturally occurs). This can translate into a scar of visibly less size, improved laxity, less separation and angle change between follicular units, reduced miniaturisation and less collagen production between the fatty tissue and dermis layer.
The process of PRP isolates the platelets from the blood, meaning a sample must be taken from the patient. The blood is then “spun” (in a centrifuge) at specific speeds to separate the plasma further, leaving the poor and rich plasma. The two important factors here are PDGF & TGF-β, platelet-derived growth factors that stimulate the replication of important stem cells for fibroblasts and endothelial cells (related to “budding” on new capillaries); stimulate the production of fibronectin, a cell adhesion molecule; and help contract and remodel wounds. TGF stimulates fibroblasts chemotaxis and the production of collagen while inhibiting collagen degradation by decreasing proteases and increasing protease inhibitors, all of which favour fibrogenesis.
Each of these factors is able to induce a unique response in improving the healing process. PDGF accelerates wound closure markedly by augmenting connective tissue matrix deposition and TGF-β stimulates new collagen deposition and maturation into larger bundles on the leading edge of the wound.
The likely effect of this is that it bypasses part of the inflammatory phase of healing. Testing is already underway on a variety of wound types and a small initial test of punch donor healing on an FUE donor showed noticeable signs of faster healing.
Dr Bisanga would like to introduce protocols that will yield the best result for patients. This will take time to test scar closure techniques, overall healing and other treatment options, yet Dr Bisanga feels that the medical benefits of the application in hair transplantation may be of significance.
Beyond just faster and improved healing; they may also affect the basics of donor management and possibly placement within miniaturised hair, to name just two possibilities.
Donor healing properties can improve future procedures by extending the number of hairs that can be extracted, decreasing miniaturisation and improving laxity.
Our Techniques
proven hair restoration results
-
FUE
FOLLICULAR UNIT EXCISION
-
FUT / STRIP
TRADITIONAL METHOD
-
BHT & REPAIR
BODY HAIR TRANSPLANT (BHFUE)
-
PRP
PLATELET-RICH PLASMA